MitchJi
10 MW
Hi,
Suitable for EV's and Grid (presumably cheap and durable) sounds very promising.
...Their battery has a specific energy high enough to challenge the specific energies of fuel cells...
http://www.greencarcongress.com/201...tteries-as-next-generation-of-high-capac.html
Suitable for EV's and Grid (presumably cheap and durable) sounds very promising.
...Their battery has a specific energy high enough to challenge the specific energies of fuel cells...
http://www.greencarcongress.com/201...tteries-as-next-generation-of-high-capac.html
greencarcongress said:Univ. of Texas researchers propose lithium- or sodium-water batteries as next generation of high-capacity battery technology; applicable for EVs and grid
Researchers at the University of Texas, including Dr. John Goodenough, are proposing a strategy for high-capacity next-generation alkali (lithium or sodium)-ion batteries using water-soluble redox couples as the cathode.
In a paper published in the Journal of the American Chemical Society, they report on their demonstration of feasibility of a such a battery having a thin, solid lithium- or sodium-ion electrolyte separating a water-soluble redox couple as the cathode and lithium or sodium in a nonaqueous electrolyte as the anode.
The cell operates without a catalyst and has high storage efficiency. The possibility of a flow-through mode for the cathode allows flexibility of the cell design for safe, large-capacity electrical-energy storage at an acceptable cost, they conclude.
Example of a lithium-water rechargeable battery. Credit: ACS, Lu et al.:
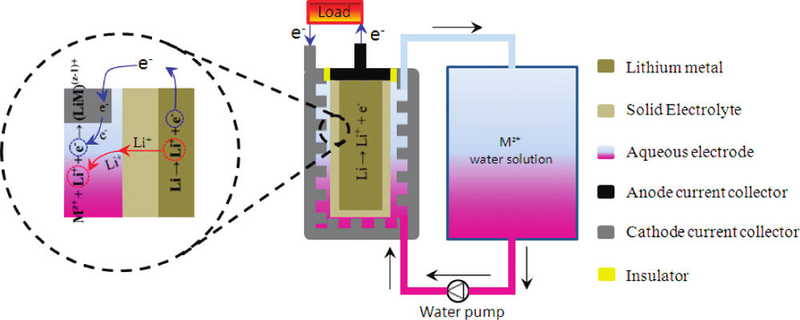
Although intense effort has been devoted to the development of carbon or carbon-buffered alloys as anodes for lithium batteries, lithium metal still has the highest specific capacity (3860 mA h g-1) and sodium metal would be cheaper; therefore, an important challenge for the third generation of alkali-ion batteries is how to fully and effectively utilize the high capacity of an alkali metal and increase the capacity of the cathode beyond what is possible with an insertion compound while operating at ambient temperature. The present sodium-sulfur battery operates above 300 °C.
Oxygen in air, an “inexhaustible resource†on earth, makes it possible to match the capacity of lithium metal. In theory, the specific energy of a lithium-oxygen (air) battery is 5200 W h kg-1. The high energy storage has stimulated a worldwide study of Li-air batteries. A typical Li-air battery discharges at 2.5-2.7 V and charges at 4.2-4.4 V. The large discrepancy of 1.7 V between the charge and discharge curves represents a low Coulombic efficiency (CE), even when expensive catalysts have been used to lower the overpotential of the oxygen reactions. Moreover, slow oxygen diffusion in the cathode degrades the performance of the Li-air battery.
In theory, the decomposition potential of water is 4.27 V vs. Li/Li+ at room temperature. The high theoretical potential predicts the possibility of obtaining a lithium-water battery with a high potential through a proper structural design. Recently, a Li|β-NiOOH (water) battery with an operating potential of ~3.4 V was developed. The cathode, β-NiOOH, exhibited a specific capacity of 256 mA h g-1, but its capacity is still far smaller than that of lithium metal. Here we present a new strategy for alkali-ion batteries using water-soluble redox couples as the cathode.
—Lu et al.
The electrochemical reactions at the electrodes are:
anode: nA → nA+ + ne–
cathode:Mz+(aq) + ne– → M(z-n)+(aq)
A = lithium or sodium (Li or Na), M represents a metal and 1 ≤ n < z. The overall reaction is written:
nA + Mz+(aq) → nA+ + M(z-n)+(aq)
Electrochemical behavior of the proof-of-concept battery. Credit: ACS, Lu et al.
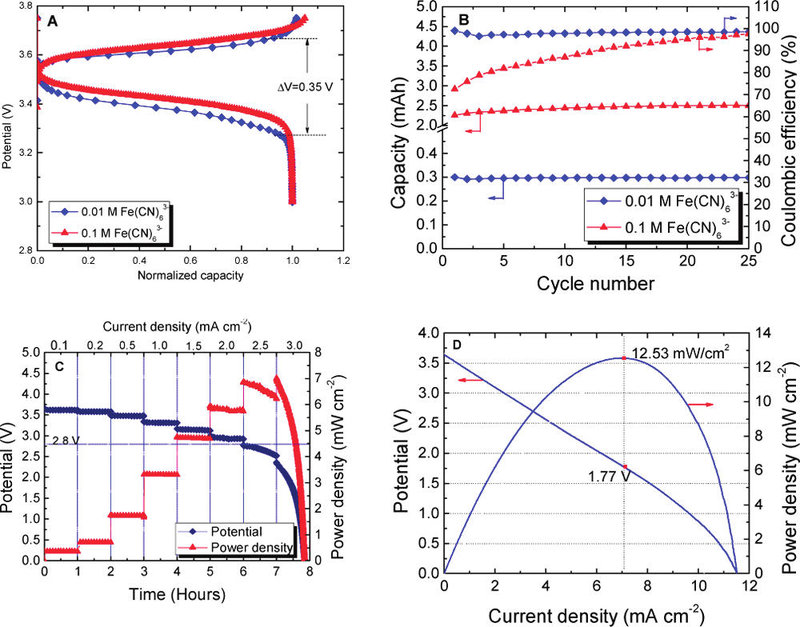
In their paper, Lu et al. note that an aqueous cathode has a low viscosity and can be easily circulated in a flow-through configuration at room temperature. The aqueous cathode could be individually stored in tank, reducing the volume of the battery and increasing the design flexibility of the battery structure. Their battery has a specific energy high enough to challenge the specific energies of fuel cells.
The flow of the aqueous solution brings heat out of the battery system and keeps the battery working near ambient conditions. Furthermore, the aqueous cathode does not suffer from H2 evolution from the solution, and the battery is efficiently rechargeable, they note.
The aqueous cathodes must:
* have proper redox potentials;
* no side reactions;
* good stability in water;
* good reversibility;
* reliable safety; and
* low cost.
In their initial proof of concept study, they used aqueous Fe- (NO3)3/Fe(NO3)2 as the cathode, with a relatively thick, commercially available lithium superionic conductor (LISICON).
...we have demonstrated the feasibility of an alkali-ion battery utilizing an alkali metal as the anode and a redox couple soluble in aqueous solution as the cathode. However, the capacity of an aqueous cathode is small...requiring the concept to be extended to a flow-through mode for the cathode. Also, sodium rather than lithium might be used as the anode. This new strategy represents a third-generation alkali-ion battery promising lower cost than the conventional lithium-ion rechargeable battery, safe operation, and a Coulombic efficiency and voltage greater than those of a Li-air battery with, in principle, a comparable capacity. The demonstrated power output was limited by the commercially available solid electrolyte separating the organic-liquid or polymer anolyte and the aqueous cathode; the need for the design of a superior solid electrolyte has been indicated. The new strategy promises to be applicable to both the electric-vehicle market and the problem of electrical energy storage for the grid.
—Lu et al.
John Goodenough. In 2009, US Energy Secretary Steven Chu named Dr. John B. Goodenough as a winner of the Enrico Fermi Award “in recognition for his lasting contributions to materials science and technology, especially the science underlying lithium-ion batteries. Dr. Goodenough, a physicist, identified and developed the cathode materials for the lithium-ion rechargeable battery that is ubiquitous in today’s portable electronic devices. This material has proven to be inexpensive, environmentally friendly, safe, sustainable, and capable of thousands of charge cycles with a constant output voltage without a loss of capacity. Batteries incorporating this cathode material are used worldwide for cell phones and other portable wireless devices, power tools, hybrid automobiles, small all-electric vehicles, as well as increasingly for electrical energy storage for alternative energy, such as wind and solar power.â€
Goodenough invented lithium cobalt oxide cathode materials while at Oxford University. His technology was used in the first commercial Li-ion battery, launched by SONY in 1991. More recently, at the University of Texas, Austin, Dr. Goodenough patented a new class of iron phosphate materials with potential to replace the more costly cobalt materials. In 2000, he received the prestigious Japan Prize for his discoveries of the materials critical to the development of lightweight rechargeable batteries.
Resources
Aqueous Cathode for Next-Generation Alkali-Ion Batteries Yuhao Lu, John B. Goodenough, Youngsik Kim Journal of the American Chemical Society doi:http://pubs.acs.org/doi/abs/10.1021/ja201118f

