But it's not that simple. In galvanic corrosion the anode gets all or most of the corrosion.It's called galvanic corrosion, and will happen between many dissimilar metals whether there is current flow or not, even without being in an electrical circuit. Faster with some combinations with others, and typically faster with higher humidity.
- anode - washers - zinc plated mild steel
- cathode - fuse - gold plated brass or copper (alloy)
But here the cathode gets significant damage too, probably even more than the anode.
Not advisable. Grease requires large bolts and high clamping forces. But as mentioned above, due to the hardware used, we are way below specs even for dry contacts.You may be able to minimize it by applying a dielectric grease (like on car battery terminals) to all of the mating surfaces before connecting them, this will prevent humidity from entering the connection and reduce the corrosion problem.
Torque vs. contact resistance goes something like this:

But if you apply grease all this graphics shifts up. And also remember - we are way below recommended torque.
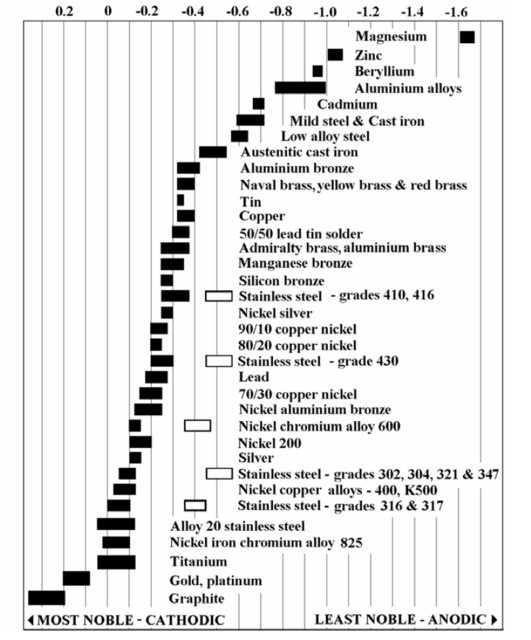
The advisable thing is to change the metals being in contact, according to the galvanic series: