safe
1 GW
- Joined
- Dec 22, 2006
- Messages
- 5,681
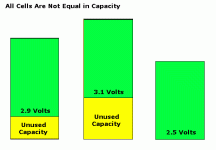
It's interesting data, but it doesn't do anything for my balancing "masters thesis". :? In order to make a mathematical argument for the "Smart Battery" I need to see the data sliced across all the cells at the same time and ideally I need to see a cross section of the cells as they discharge. The idea is to be able to prove that a group of cells that are controlled independently can produce more power if optimized than the losses that MOSFET resistance adds to the equation.
My "mathematical intuition" suggests that balancing is philosophically flawed and can be improved upon by "Smart Battery" theory. (note: that's the "On State" or "Off State" idea)
Well... I guess my Ph.D will have to wait... :lol:
My "mathematical intuition" suggests that balancing is philosophically flawed and can be improved upon by "Smart Battery" theory. (note: that's the "On State" or "Off State" idea)
Well... I guess my Ph.D will have to wait... :lol: